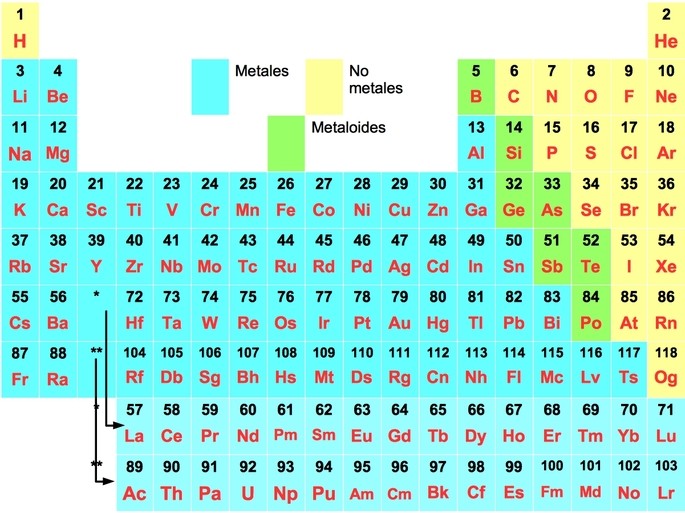
Chemical elements are classified into metals and nonmetals. Metals are substances that conduct electricity, can form sheets or wires, and have luster. Non-metals are all those substances that do not conduct electricity, are fragile to handling or are gases.
The main characteristic that differentiates metals from nonmetals is the ability to conduct electricity.
There are elements that look like metals, but react like nonmetals. These elements are known as metalloids and are boron, silicon, germanium, arsenic, antimony, tellurium and polonium.
| Metals | Non-metals | |
|---|---|---|
| Definition | Elements that have a tendency to lose electrons and conduct electricity. | They are the elements with a tendency to gain electrons and bad conductors of electricity. |
| Characteristics |
|
|
| Examples |
|
|
Of the 118 elements in the periodic table, metals account for 80% of the elements. Here are the elements of the periodic table and their corresponding classification as metals, nonmetals and metalloids:
What are metals
Metallic elements are those that have a tendency to give up electrons and can conduct electricity. They also have a characteristic luster, are malleable and ductile.
They are located on the left-hand side of the periodic table, with the leftmost elements having the most metallic character.
Metals can be classified into:
- Alkali metals are the elements lithium, sodium, potassium, rubidium, cesium and francium.
- Alkali earth metals are the elements beryllium, magnesium, calcium, strontium, barium, and radium.
- Transition metals are those found in the central block of the periodic table, and include copper, gold, silver, platinum, among others.
Characteristics of metals
- They conduct electricity: they allow the movement of electrons through the material.
- Their valence electrons move freely.: the electrons in the outermost shell of metals can move freely.
- They are prone to lose electronsWhen metals react, they usually lose electrons.
- They are reactive: the chemical reactivity of metals increases going down in the group, but decreases along the period.
- They are joined by metallic bondsmetals establish metallic bonds between themselves, a sea of electrons that “walk” between the nuclei of the atoms.
- They are solid at room temperatureMost metals are solids, with the exception of mercury Hg, gallium and cesium which are liquid at room temperature.
Examples of metals
Aluminum
It is the most abundant metal in the earth’s crust with a silvery-white luster, light, whose atomic number is 13. It is a solid that melts at 660 °C. Aluminum is extracted from bauxite, a form of hydrated aluminum oxide.
It is widely used in cookware and industrial applications because of its high resistance to corrosion.

Barium
It is a metal of atomic number 56, belonging to the alkaline-earth metals. It is a solid that melts at 727 ºC, discovered in 1808 by Humphrey Davy. It is obtained from barite, a barium sulfate salt.
Barium in pure form has a silvery appearance like lead.
Beryllium
It is the fourth element of the periodic table, belonging to the alkaline-earth metals. It is a solid that melts at 1287 ºC and is found in the composition of emeralds and aquamarines.

Bismuth
It is a brittle, crystalline, white metal, with atomic number 83, long mistaken for tin or lead. It is the most diamagnetic of the metals and has the lowest capacity to transmit heat. Bismuth is used to make alloys to coat objects that can be damaged at high temperatures, such as fire detection equipment or fire extinguishers.
Calcium
It is the fifth most abundant metallic element in the earth’s crust. It has a silvery color, is a hard solid, with atomic number 20. It is a member of the alkali earth metals and is a constituent of important biological structures such as bones, teeth and shells.
Cesium
It is the most electropositive and alkaline metal. It is found in a liquid state at room temperature, along with gallium and mercury. Caesium explodes on contact with water and has a high affinity for oxygen. It is used in atomic clocks.
Chromium
It is one of the transition metals, with atomic number 24, a solid that melts at 2671 ºC. Chromium is used to harden steel, to give it a hard, shiny surface to prevent corrosion and in the manufacture of glass to give it a green color.
Copper
Copper is one of the most important metals for human beings, who have been using it for more than 5000 years. Its reddish metallic luster stands out in addition to being malleable and ductile, which allows its use in the construction of jewelry, kitchen utensils and electrical supplies.

Iron
It is the most common metal on Earth, as it forms a large part of the planet’s core. Its atomic number is 26, it is a hard, brittle, solid that melts at 1538 ºC. Iron is part of the oxygen transport systems in living organisms. In its pure state, iron is very reactive and corrodes rapidly in humid environments.
Gold
It is the most malleable and ductile metal, of great beauty in its pure state. Its atomic number is 79 and it belongs to the transition metals. It is a good conductor of electricity and heat and resistant to corrosion. It was used as currency and is now the standard in the monetary system of many countries.

What are non-metals
Non-metallic elements are all those elements that do not fit into the characteristics of metals. Non-metals include halogens, noble gases, hydrogen, carbon, nitrogen, phosphorus, oxygen, sulfur and selenium.
They are located on the right side of the periodic table, separated from the metals by the metalloids.
Characteristics of nonmetals
- They do not conduct electricityNon-metallic elements are poor conductors of electricity.
- Their valence electrons are restricted: the electrons of nonmetals are more restricted in their motion.
- They are prone to gain electrons: atoms of non-metallic elements when reacting tend to accept electrons from other elements.
- They have reactivity: the chemical reactivity of nonmetals decreases in the group, but increases in the period.
- They form covalent bonds with other nonmetals.Non-metallic elements tend to share their electrons in the valence shell with other non-metals, forming covalent bonds.
You may also be interested in Types of chemical bonds.
Examples of non-metals
Bromine
It is the only non-metallic element found as a reddish-brown liquid at room temperature. Its atomic number is 35 and it belongs to the halogen group. It is used to make fireproofing agents, water purifiers, dyes, medicines and disinfectants.
Carbon
It is the non-metallic element with the greatest capacity to combine with other elements, key to the construction of biological molecules. It is found free in nature as diamond and graphite. In the atmosphere it is found combined with oxygen as carbon dioxide.

You may be interested to see also Examples of organic and inorganic compounds.
Chlorine
It is a yellow-greenish gas, with atomic number 17, belonging to the halogen group. It is found in nature combined with other elements, such as the common salt NaCl. Chlorine compounds are widely used throughout the world as disinfectants, bleaches, water purification, among others.
Fluorine
It is the most electronegative and reactive element, it belongs to the halogen group, with atomic number 9. It is a pale yellow corrosive gas in its pure state.
Helium
It is the second element of the periodic table and first of the noble gas group. Its name is derived from the Greek helios meaning “sun”, where it was first identified in 1868. Along with hydrogen, it is the most abundant element in the entire Universe.
You may be interested to see also:
References
Lide, D. R. (editor) (2005). CRC Handbook of chemistry and physics, CRC Press, Boca Raton, Florida.
Vernon, R. E. (2013). Which elements are metalloids? Journal of Chemical Education 90:1703.